Corrosion is the tendency in metals to return to their combined or natural state in the form of oxides, hydroxides and salts.
Corrosion can also be defined as a destructive attack on a metal due to processes of electro-chemical oxidation in the natural ambient environment.
Electro-chemical corrosion is what takes place in metals when they are surrounded or immersed in a conductor of current called electrolytes. In the case of boats, seawater is the electrolyte. Seawater is capable of conducting current to specific zones or metals of a boat. These metals that are electrically interconnected tend to have zones with different electric potentials: cathodes and anodes that provoke corrosion.
The current flows from the anode to the cathode and the metal that receives the current (that which acts as a cathode), is protected.

Rudder of a boat that was unprotected
Cathodic protection of a specific metal consists of lowering the potential (greater negative reading) of the metal to be protected below the defined value for that metal. That level is defined as a thermodynamic point where corrosion cannot occur. Below this defined level, electro-chemical corrosion cannot occur.
2 techniques exist for lowering this potential:
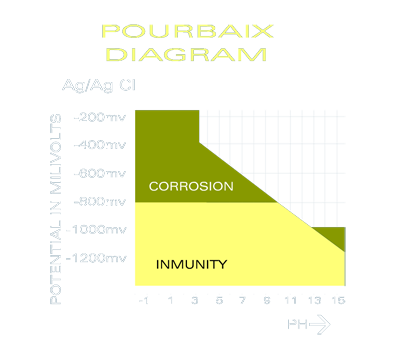
The "Pourbaix" diagram based on a submerged steel plate indicates that the metal should have a potential (or reading) of below -800mV. This is measured with a silver reference electrode. If the boat is made of aluminum the minimum potential should be below -900mV and in the case of bronze/stainless steel boats the reading should be below -700mV.
The Proytec anodes are made of Titanium and undergo a series of modifications and once activated, can be used commercially. The activation of the Titanium gives it properties that are very conducive to transmitting current into the water.
Through use of the Titanium anodes we can transmit continuous high intensity current without having to rely on imprecise and unreliable AC transformers. For this reason Proytec equipment has no maintenance. Additionally, Titanium anodes have a useful life of 20 years, unlike sacrificial anodes that need to be replaced every year.
The Proytec system also protects the environment because it does not contaminate the water with heavy metals. Traditional protection systems using sacrificial anodes such as zinc (or any other metal) are dissolved in the electrolyte (seawater) during their operation.
All these features show that the Proytec system is the most reliable and has the best relationship between quality and price of solutions available. Compared to zinc protection systems, the Proytec system will amortize itself within 4-5 years.